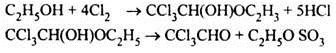Although essential for controlling pests, little is realised that once exposed to environment, their post application fate is subject to environment forces causing them to move from target to non-target species and area during which they undergo myriads of transformations sometimes cause unforeseen environmental problems.
The pesticides can translocate from the point of application to non-target components of environment often creating problems. They also undergo a variety of transformations by biotic and abiotic agents mostly breaking down into small non-toxic products.
However, a few of these transformation products are more toxic and their eco-toxic significance ought to be assessed seriously if safe use of pesticides is to be ensured. On the other hand some good pesticides undergo rapid breakdown and the consequent loss of efficacy is a matter of economic concern.
A knowledge of these processes is, therefore, very essential to judge the environmental impact and ecotoxic significance of pesticides used in agriculture and public health and to take appropriate remedial measures to ensure safe use of pesticides.
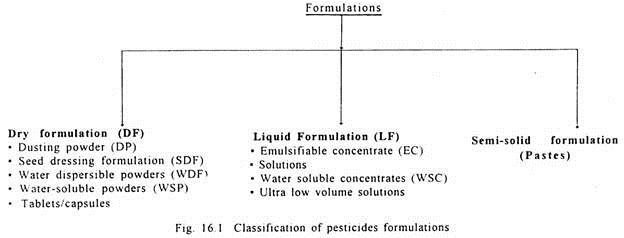
Pesticides include insecticides, fungicides, weedicides, rodenticides, nematicides and plant growth regulants. The liquid formulations include emulsifiable concentrates (EC), solutions water soluble concentrates (WSC) and ultra-low volume solutions. There are also some smoke-generating pesticides which when dissolved in a highly combustible chemical, forms dense white smoke. These classifications are shown in Fig. 16.1.
Classification of Pesticide Formulation:
Details of these formulations are given below:
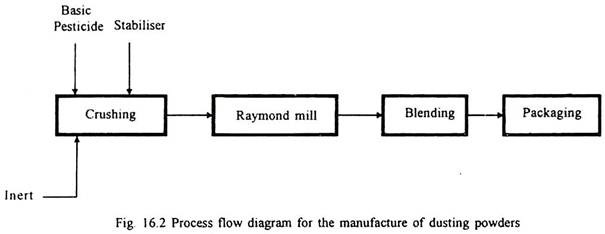
1. Dusting Powders (DP):
The basic pesticides are mixed with a powdered mineral base, such as soapstone, lavigated clay and hydrated calcium silicate (called inert). Certain auxiliaries, such as kaolin, lampblack, dicalcium phosphate, zinc oxide etc., are added in very small quantities so as to overcome the problem of formation of balls which makes application through dust sprayers difficult. The manufacturing process involves crushing, pulverising, blending and packing as given in Fig. 16.2.
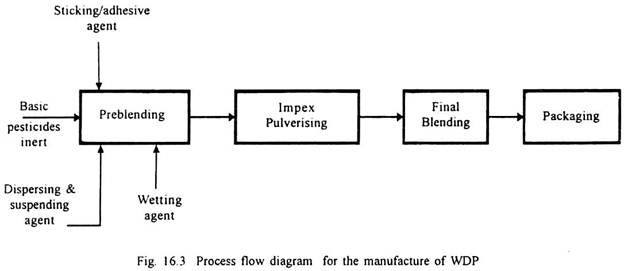
2. Water-Dispersible Powders (WDP):
The water-dispersible powders are finely divided pesticide dust concentrates which contain surface active agents that will allow the concentrate to be diluted to required strength to form stable sprayable suspensions. Inerts used are lavigated clay and hydrated calcium silicate.
Carboxy methyl cellulose is used as dispersing and suspending agent. Sodium salt of alkyl aryl sulphonate is used as wetting agent, and as sticking or adhesive agent, animal or vegetable oil glue is used. Process includes preblending, pulverising, postblending and packing as given in Fig. 16.3.
3. Granules:
The granules formulation consists of absorptive granular carrier (clay) containing solution of basic pesticide adsorbed on the surface. Calcium silicate is used as an absorbent. The granules need no further dilution and have easier application, thereby reducing hazards to the operators.
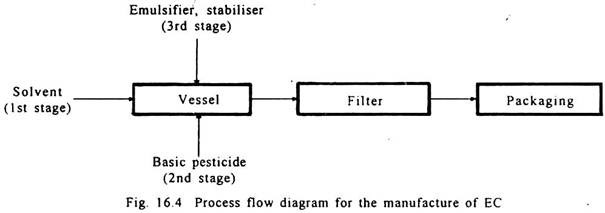
4. Emulsifiable Concentrates (EC):
The emulsifiable concentrates have three components consisting of basic pesticide, solvent and emulsifier. Sometimes a stabiliser is also used. These concentrates give emulsions when diluted with water (Fig. 16.4).
Solvents used are aromex, butanol, cyclohexane and xylene. Emulsifiers used are polyoxyethylene, ether, alkyl aryl sulphonate. Stabilisers used are epichlorohydrine, triethanolamine and urea.
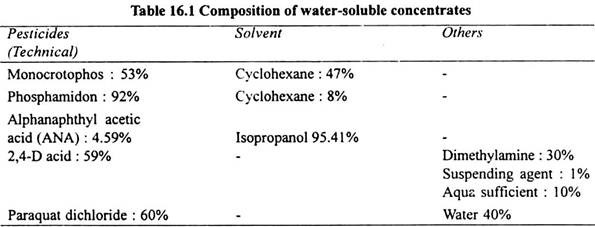
Water-Soluble Concentrates (WSC):
The water-soluble concentrates contain wetting agent which on dilution forms a clear solution that can be sprayed on the target. The composition of various WSC formulations is given in Table 16.1.
Technical Pesticides:
Manufacturing process of some of the major technical grade pesticides is discussed below. The raw materials used and products manufactured in each of the 57 large/medium scale pesticides industry were furnished in the document ‘Pesticides Industry – Status’ of the Central Board.
Diphenyl Dichloro Triochloroethane (DDT):
The technical grade of DDT is a mixture of two isomers known as P, P [1-trichloro-2, 2-bis (p. chlorophenyl] ethane) and O, P (1-trichloro-2-O, chlorophenyl-2-P chlorophenyl ethane). The former is the main constituent.
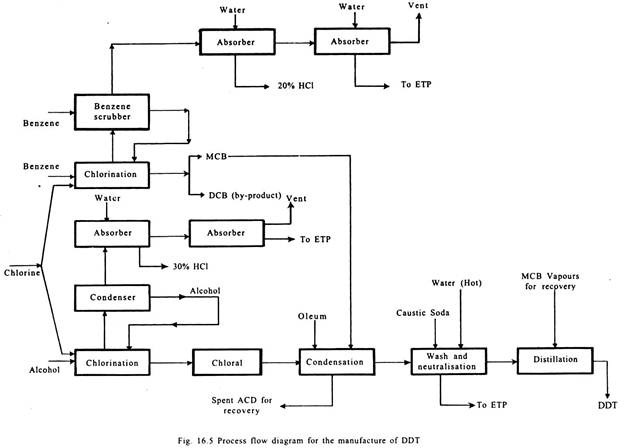
The process of manufacture consists of the following steps:
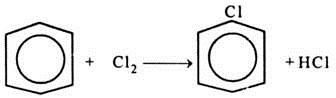
(i) Manufacture of Monochloro Benzene (MCB) through chlorination of benzene using iron as catalyst;
(ii) Manufacture of Chloral through chlorination of ethyl alcohol;
(ii) Manufacture of DDT through condensation reaction between MCB and chloral using oleum as condensing agent; and
(iv) Recovery of by-products such as dilute HCl, dichlorobenzene (DCB), and sulphuric acid. Process flow chart is given in Fig. 16.5.
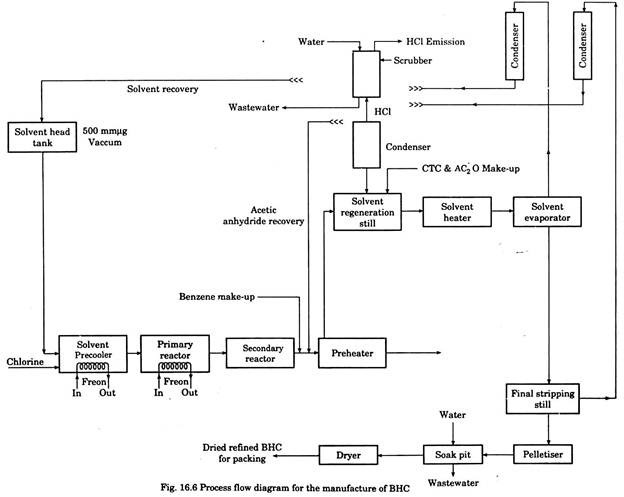
Benzene Hexachloride (BHC):
BHC is manufactured by the reaction of benzene and chlorine in the presence of ultraviolet radiation and a solvent medium consisting of benzene, acetic anhydride and carbon tetrachloride.
After the completion of chlorination reaction, the excess solvents are separated and recovered for reuse by a distillation step. The molten BHC is quenched in water in a pelletiser where pellets are formed.
These pellets are discharged to a soaking tank for washing unreacted solvents. It is then dried in a drier. Process flow diagram is given in Fig. 16.6.
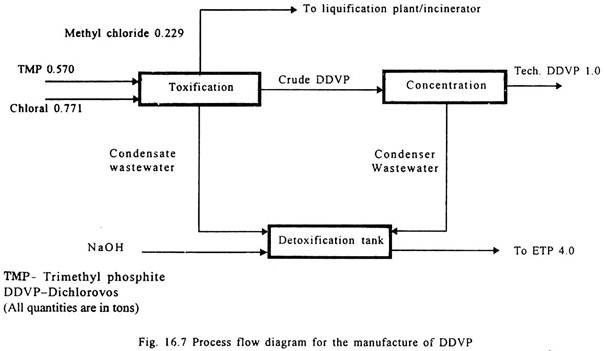
Dichlorovos (DDVP):
Anhydrous chloral and trimethyl phosphite are added simultaneously for the reaction to take place in a crude DDVP medium during which temperature is controlled by chilled water cooling.
The simultaneous addition of the reactants is highly essential as, otherwise, side reactions would take place. After the completion of the reaction, the reaction mixture is degassed to remove methyl chloride gas. The product is further purified. Methyl chloride is recovered as a by-product.
It is also manufactured by condensation of dichloro acetaldehyde and trimethyl phosphite, and the temperature is controlled using chilled water cooling. The product is then concentrated. Process flow diagram is given in Fig. 16.7.
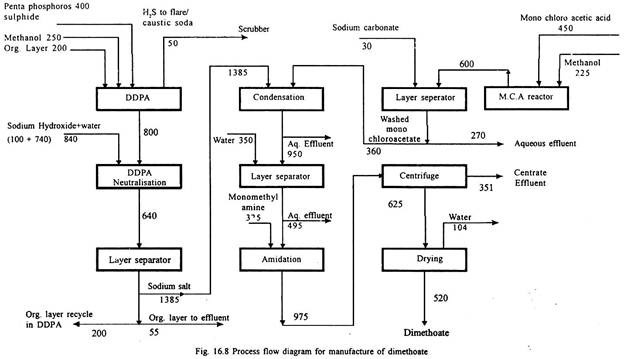
Dimethoate:
Phosphorous pentasulphide is reacted with methyl alcohol to form dimethyl dithio phosphoric acid (DDPA) and hydrogen sulphide gas. DDPA is neutralised with caustic soda to form sodium salt of DDPA.
The organic phase forms effluent. Methyl chloro acetate is manufactured by reacting monochloro acetic acid with methanol and condensing with sodium salt of DDPA. This condensed product is by reacting with monomethyl amine to produce dimethoate. Process flow diagram is given in Fig. 16.8.
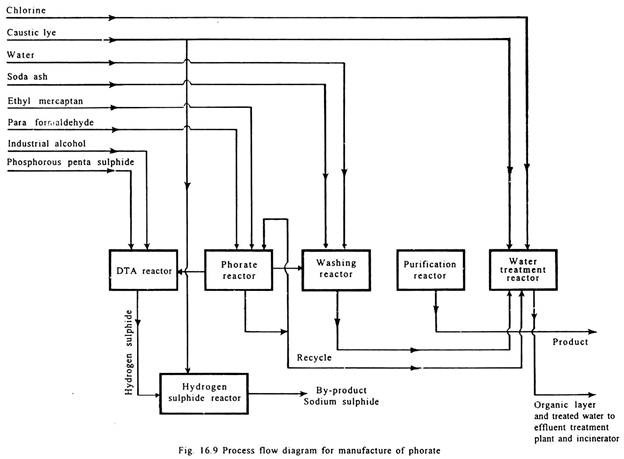
Phorate:
Phosphorous pentasulphide is reacted with ethanol to produce diethyl dithiophosphoric acid which is further reacted with formaldehyde and ethyl mercaptan to form crude phorate.
It is then purified. Hydrogen sulphide gas evolved is scrubbed with caustic to form sodium sulphide, a by-product. Process flow diagram is given in Fig. 16.9.
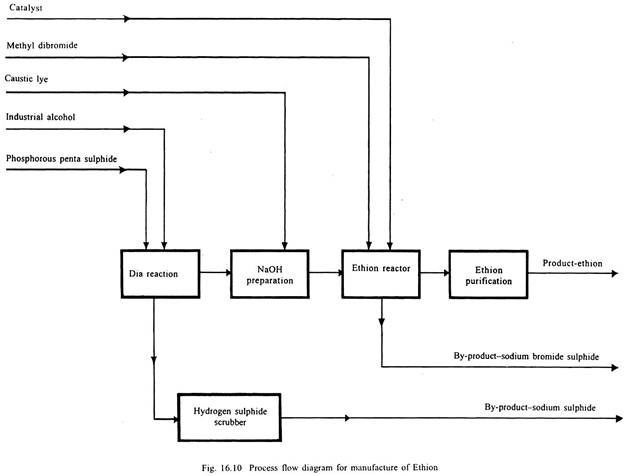
Ethion:
Phosphorous pentasulphide and absolute alcohol are reacted to produce diethyl dithiophosphoric acid (DTA), which is reacted with caustic to form sodium salt of DTA. This salt and methylene dibromide are reacted together to produce ethion. Process flow diagram is given in Fig. 16.10.
Monocrotophos:
Monomethyl acetoacetamide is chlorinated in the presence of solvent at low temperature. The organic layer is condensed with trimethyl phosphite to form monocrotophos.
Malathion:
It is manufactured by the condensation reaction of dimethoxy dithiophosphoric acid (DTA) and diethyl maleate (DEM) in the presence of hydroquinone.
DTA is produced by reaction between phosphorous pentasulphide and methanol in toluene phase. Hydrogen sulphide gas obtained in the reaction is scrubbed with caustic soda to form sodium sulphide, a by-product.
DEM is produced by reaction between ethanol and maleic anhydride in the presence of benzene using sulphuric acid as catalyst.
Phosphamidon:
Diethylacetoacetamide (DEAA) is chlorinated using chlorine. HCl gas removed by degassing is scrubbed to obtain HCl as by-product. Reaction mass is neutralised using sodium carbonate, and dichloro diethyl acetoacetamide (DCAA) present in the organic layer is removed.
DCAA is diluted in xylene, heated to 100°C and trimethyl phosphite is added. From the reaction mass, methyl chloride gas is removed by degassing and phosphamidon obtained.
Cypermethrin:
Methyl ester is hydrolysed by caustic solution in the presence of methanol. After hydrolysis, methanol is recovered by distillation and the remaining material is acidified by adding hydrochloric acid.
The organic layer is separated and is chlorinated by using thionyl chloride and hexane, and condensed with metaphenoxy benzaldehyde and sodium cyanide to get cypermethrin. Hexane is recovered and the aqueous layer forms effluent.
Fenvalerate:
Parachloro toluene (PCT) is chlorinated with chlorine and the reaction mass is further treated with sodium cyanide. Water is added to the reaction mass and aqueous layer is drained to ETP.
The organic layer, after recovery of excess PCT, is treated with isopropyl bromide and caustic soda flakes. Water is added and aqueous layer is drained to ETP. The organic mass is acidified with sulphuric acid after adding toluene and water, and distilled to obtain fenvalerate.
Quinalphos:
First step involves manufacture of quinoxalinol. Acetic acid is reacted with chlorine to yield monochloroacetic acid and then its sodium salt (NaMCA) is prepared adding soda ash. By condensation reaction of NaMCA and O-phenylenediamine, dihydroxy quinoxalinol is obtained.
This is oxidised to give sodium salt of quinoxalinol which is isolated by adding HCl to yield quinoxalinol. Quinalphos is formed by the condensation reaction of quinoxalinol and diethyl thiophosphoryl chloride. It is further processed to obtain required purity.
Phosalone:
Orthoaroinophenol is reacted with urea in solvent medium to get benzoxazolone, which is then chlorinated in solvent medium, and formaldehyde is added to get hydroxy methylchloro benzoxazolone (HMCB).
MCB is chlorinated using dry HCl gas to get chloromethyl ehlorobenzoxazolone (CMCB) which is extracted in methylene chloride medium. P2S5 is reacted with ethyl alcohol to get diethyl dithiophosphoric acid (DEDTP) which is neutralised with caustic soda to get sodium salt of DEDTP. This sodium salt and CMCB are reacted to get crude phosalone.
The storage levels of DDT and DDE in people in other countries have also been investigated. The levels were lowest in countries where little DDT is used, e.g. Denmark with DDT 0.6 and DDE 2.7 ppm and highest in countries such as India, DDT 16 and DDE 10 ppm, where large amounts of DDT are used in malaria eradication.
The storage of DDT in human fat is a direct function of level of intake and is lowest in meat abstainers, DDT 2.3 and DDE 3.6 ppm; and Eskimos, DDT 0.8 and DDE 2.2 ppm; whose diets contained the lowest amounts of DDT.
Volunteers given 35 mg per day orally stored DDT 281 and DDE 40 ppm, and a formulator in a DDT factory had DDT 648 and DDE 483 ppm; yet these men remained in good health. The half-life of DDT and DDE stored in humans is about 0.5 years so that in the absence of DDT intake the levels in human populations should slowly decline.
DDT and its metabolites DDE and DDD are also found in most other human tissues – blood DDT 0.0068 and DDE 0.0114 ppm, milk 0.08-0.13 ppm, adrenal glands 0.7 ppm. DDT ingested by humans is excreted slowly as 4, 4-dichlorodiphenyl acetic acid (DDA) which is present in the urine of the general U.S. population at <0.02 – 0.18 ppm. The levels of DDA in human blood and urine roughly parallel the rate of intake and can be used to monitor the exposure.
The significance of levels of DDT and its metabolites DDE and DDD in the general population is complex. Food is generally believed to contribute as much as 89% of the total intake of DDT but recent studies showing substantial fat storage of DDT in Eskimos and in institutional patients where DDT intake is very low suggest that non-dietary sources such as house dust may contribute upto 50% of the total body burden.
The non-white population of the U.S. has significantly higher fat storage of DDT than the white population suggesting the influence of dietary factors and socio-economic factors resulting in greater use of household insecticides.
Fat storage of DDE increases with the age of the population, especially in non-whites where the level increased from 4.06 ppm in the 0-5 years category to 8.61 ppm at 41-50 years and 15.50 ppm at 90+ years.
Accumulation in States with cooler climates averaged 4.85 ppm as compared with 9.21 ppm in warmers states. There is no clear cut evidence of a sex difference in fat storage of DDT or in positive association with specific disease conditions.
Other Pesticides in Humans:
Other stable organochlorine insecticides or their stable metabolites are also found in human tissues. Surveys of the population have shown levels of these materials in fat and blood—BHC isomers—fat 0.20-0.60 ppm, blood 0.0031-0.0019 ppm, heptachlor epoxide—fat 0.10—0.24 ppm, blood 0.0008 – 0.0011 ppm.
In addition to these, pesticides demonstrated in the blood of the general population, trace levels of chlordane, toxaphene, endrin, aldrin, pentachlorophenol and 2,4-D have been found in tissues of humans with unusual occupational or accidental exposure.
Most of the other types of pesticides widely used, the organophosphorous esters, carbamates, phenoxy and benzoic-acids, triazines, ureas are less persistent in the environment than the organochlorine compounds and are rapidly metabolised by enzymes in the human liver and converted to predominately water-soluble products eliminated in the urine.
Therefore there is little evidence of fat storage of these compounds. Monitoring of the urinary content of water-soluble metabolites is a very sensitive measure of intake of such pesticides and has been studied for parathion, malathion and related organophorus esters, the carbamates carbaryl and propoxur, dintro-o-cresol, lead, arsenic and mercury.